California Board of Pharmacy Compounding and Hazardous Drugs Regulations – Monitoring Positive and Negative Pressure
Compliance for Compounding Pharmacies
All compounding pharmacies will be required to comply with the pending general regulatory updates in 16 CCR 1735 and 16 CCR 1751 on January 1, 2017. The California compounding regulations have since been finalized, and can be found here: https://www.pharmacy.ca.gov/laws_regs/1735_ooa_clean.pdf.
As of January 1, 2017, all California sterile compounding pharmacies must be compliant with the most recent laws and regulations. Let’s look at some of these regulations and see how to ensure your sterile compounding pharmacy meets (or exceeds) state guidelines. The specific regulations pertain to monitoring and recording positive and negative pressure as well as temperature and relative humidity (RH):
- Section 1735.1(a)
- Section 1735.1(j)
- Section 1735.1(l)
- Section 1735.6(2)
- Section 1751.(4)
- Section 1751.1(6)[a,b,c]
- Section 1751.1(8)
- Section 1751.1(11)[c]
- Section 1751.3(21)
The above sections outline the proper monitoring and recording of negative and positive air pressure as well as general environmental monitoring and the recording of these variables.
A typical clean room consists of an anteroom (or gowning room) and a sterile room (cleanroom). Maintaining the correct air pressure differential between these two rooms is critical in a compounding pharmacy environment for the safety of cleanroom workers as well as related staff.
Monitoring air pressure and controlling air pressure based on the data monitored are separate initiatives. A California State-compliant clean room monitoring system should include the following:
- Ability to monitor negative and positive air pressure differentials between multiple areas of interest.
- Chart and record collected monitoring data for quick and effortless retrieval upon state inspection.
- Store logged data for prolonged periods of time in an integrated/on-board storage array.
- Allow for fast and organized download of collected negative and positive air pressure differential readings.
- Have the ability to sample in air pressure differential in intervals set by cleanroom administration, and has the ability to change based on sterile compounding needs.
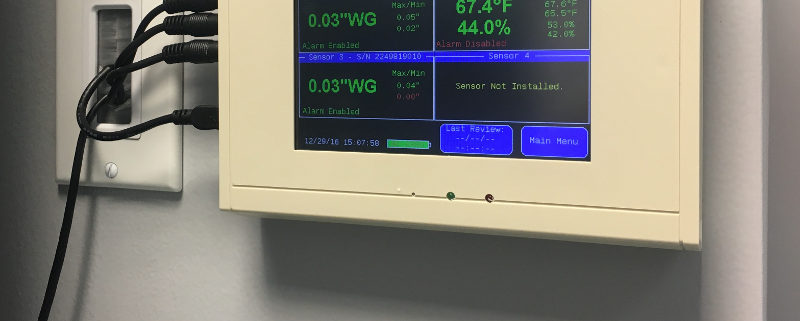
- The ability to chart, and log, temperature (±.05°F), relative humidity (RH) (±3%), and differential pressure (±0.007″ WC)
- Additional advantageous accessories to the ideal system would include the ability to send alert notifications via text/SMS to all pharmacy personnel, or phone call alerts with an automated phone dialer. Having an audible room alarm would be a plus.
In most cases, current technological hurdles prevent compounding pharmacies from possessing an “all-in-one” solution. Buying separate system components to monitor all required variables can be expensive and difficult to integrate.
Solutions
While there are many stand-alone products which, when used in combination, can provide complete compliance, it is preferred in most compounding pharmacies to have a single differential air pressure, temperature and relative humidity monitoring solution. There are very few reputable manufacturers who can accomplish this, and even fewer that offer automatic data logging, remote sensors and USA-based manufacturing, R&D, woith live product support. In fact, a company called “Two Dimensional Instruments, LLC is the only company which offers such a solution. This site (prodataloggers.com) is the only authorized distributor of the TV2 Room Pressure Monitor.
TV2 Room Pressure Monitor- An Introduction

The TV2 Room pressure Monitor is the only all-in-one monitoring solution for positive and negative air differential pressure monitoring, temperature and relative humidity monitoring, automatic data logging, with advanced and automated alerts and USA-based manufacturing & support.
The TV2 Room Pressure Monitor features some of the most comprehensive and secure data acquisition, monitoring and reporting capabilities available. The core chipset/brain of the TV2 Room pressure Monitor is in use and trusted by some of the world’s most highly technical (and demanding) companies such as NASA, Boeing, Northrup Grumman, Lockheed Martin, GE and the Department of Homeland Security.
In it’s second hardware revision and tenth software revision, the TV2 platform is a trusted workhorse in the field of aerospace, academia and other institutions where highly-accurate instrumentation is required. The TV2 Room Pressure Monitor exceeds all California Board of Pharmacy requirements concerning differential air pressure, temperature and relative humidity monitoring. Additionally, the TV2 satisfies all data logging and records-keeping requirements with 21-CFR-11 encrypted data storage.
Other features of the TV2 Room pressure Monitor can be found here: TV2 Room pressure Monitor Features
A product spec sheet can be found here: TV2 Room Pressure Product Spec Sheet
If you want to learn more about an example installation, you can view installation notes here https://www.prodataloggers.com/clean-room-installation-guide/
Finally, if you are considering other brands, see how the TV2 Room Pressure Monitor stacks up against the competition here.
If you want pricing information or to ideas in configuring your product, please visit our store!